In this section we publish best tasks from the previous IChO in Moscow held in 1996. Solution follows text of the problem with a two weeks gap. The tasks will be changed once a month. Have a nice time!
Moscow, 1996. Organic chemistry
Stereochemistry of organic compounds can sometimes be determined by studying their chemical behavior. The stereochemical configuration of one of the isomers of 5 nonbornene 2,3 dicarboxilyc acids (compound X)
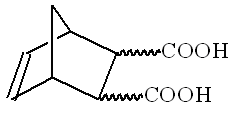
(no stereochemistry is shown) was established by the following experiments.
On heating this substance decomposes producing water and a new compound Y. Compound Y slowly dissolves in excess of aqueous NaOH with the formation of product X1 same to that is formed in the reaction of X with NaOH. The resulting solution of X1 is treated by I2 to give compounds containing iodine. Acidification of the solution leads to a mixture of two isomeric compounds, A and B in the 3:1 ratio. The titration of 0.3913 g of compound A by 0.1000 M aqueous solution of NaOH in the presence of phenolphthalein takes 12.70 ml of alkali. The same amount of 0.1000 M solution of NaOH is required for the titration of 0.3913 g of compound B. On heating compound A slowly transforms into compound C, which contains no iodine and is able to react with water. Under the same conditions compound B does no undergo this transformation, but on heating with hydrochloric acid slowly transforms into A.
All reactions must be written as balance equations. No mechanisms is required.
1. Mark by asterisks (*) the asymmetric carbon atoms in the structure of 5 nonbornene 2,3 dicarboxilyc acids.
2. Draw the stereochemical formulas of all stereoisomers of compound X, and the structures of products of their dehydration in those cases when it is possible.
3. Write the reactions of NaOH with a stereoisomer of X and a stereoisomer of Y.
4. Calculate the molecular mass of compound A. Write the reactions leading from X1 to A.
5. Write the reaction of the formation of C from A and the reaction of C with water.
6. Draw the stereochemical formula of compound X, which satisfies all of the data given in the problem.
7. Write the reactions leading from B to A.
8. Are the compounds A and B diastereomers?


